Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio. Gas stoichiometry is the quantitative relationship between reactants and products in a chemical reaction with reactions that produce gases. Gas stoichiometry applies when the gases produced are assumed to be ideal, and the temperature, pressure, and volume of the gases are all known. Often, but not always, the standard temperature and pressure are taken as 0 °C and 1 bar and used as the conditions for gas stoichiometric calculations.
Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combustion. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products and reactants that are produced or needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry.
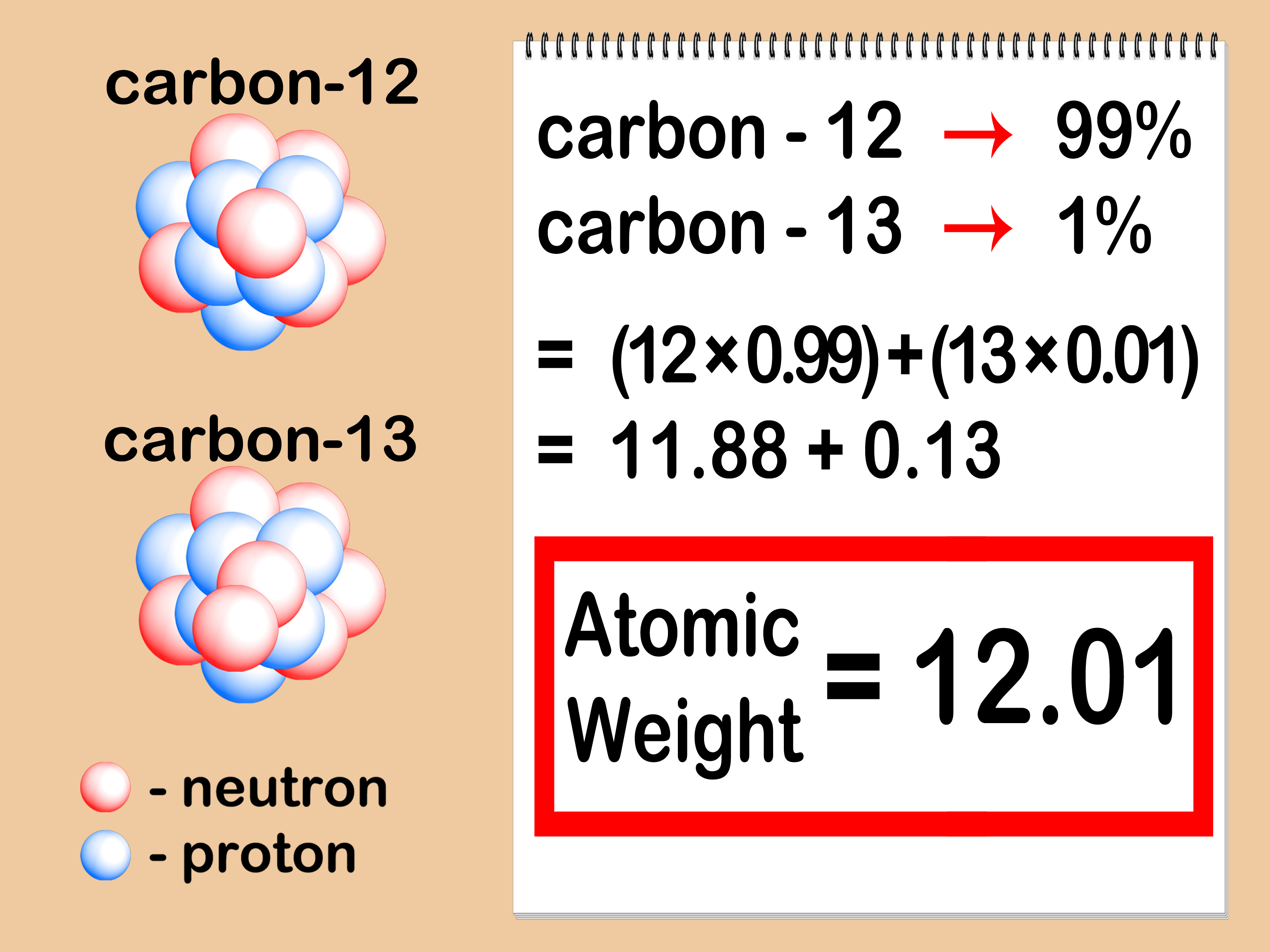
In the example above, reaction stoichiometry measures the relationship between the quantities of methane and oxygen that react to form carbon dioxide and water. In a balanced chemical equation, the coefficients can be used to determine the relative amount of molecules, formula units, or moles of compounds that participate in the reaction. The coefficients in a balanced equation can be used as molar ratios, which can act as conversion factors to relate the reactants to the products. These conversion factors state the ratio of reactants that react but do not tell exactly how much of each substance is actually involved in the reaction. Different elements have a different atomic mass, and as collections of single atoms, molecules have a definite molar mass, measured with the unit mole (6.02 × 1023 individual molecules, Avogadro's constant).
Thus, to calculate the stoichiometry by mass, the number of molecules required for each reactant is expressed in moles and multiplied by the molar mass of each to give the mass of each reactant per mole of reaction. The mass ratios can be calculated by dividing each by the total in the whole reaction. Chemical equations are symbolic representations of chemical reactions.
In a chemical equation, the reacting materials are written on the left, and the products are written on the right; the two sides are usually separated by an arrow showing the direction of the reaction. The numerical coefficient next to each entity denotes the absolute stoichiometric amount used in the reaction. In the above equation, the elements present in the reaction are represented by their chemical symbols. Displaying each element is important when using the chemical equation to convert between elements. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures.
We will consider the key developments in individual relationships , then put them together in the ideal gas law. Another method of determining the limiting reagent involves the comparison of product amounts that can be formed from each reactant. This method can be extended to any number of reactants more easily than the previous method.
Again, begin by balancing the chemical equation and by converting all the given information into moles. Then use stoichiometry to calculate the mass of the product that could be produced for each individual reactant. The reactant that produces the least amount of product is the limiting reagent. The reacting materials are given on the left, and the products are displayed on the right, usually separated by an arrow showing the direction of the reaction. The numerical coefficients next to each chemical entity denote the proportion of that chemical entity before and after the reaction. The law of conservation of mass dictates that the quantity of each element must remain unchanged in a chemical reaction.
Therefore, in a balanced equation each side of the chemical equation must have the same quantity of each element. Stoichiometry is defined as the number before the chemical formula in a balanced reaction. The stoichiometry is needed to reflect the ratios of molecules that come together to form a product. The good thing about this calculator is that it can be used any way you like, that is to find the mass of reactants needed to produce a certain mass of your product. All this information is hidden in the moles, which can be derived from a solutions molarity or concentration. The ideal gas law specifies that the volume occupied by a gas depends upon the amount of substance as well as temperature and pressure.
Standard temperature and pressure -- usually abbreviated by the acronym STP -- are 0 degrees Celsius and 1 atmosphere of pressure. Parameters of gases important for many calculations in chemistry and physics are usually calculated at STP. An example would be to calculate the volume that 56 g of nitrogen gas occupies. The law of conservation states that the quantity of each element does not change over the course of a chemical reaction. Therefore, the chemical equation is balanced when the amount of each element is the same on both the left and right sides of the equation. Next, convert all given information into moles, and compare the mole ratios of the given information to those in the chemical equation.
On some occasions, it may be necessary to calculate the number of moles of a reagent or product under certain reaction conditions. To do this correctly, the reaction needs to be balanced. The law of conservation of matter states that the quantity of each element does not change in a chemical reaction. Therefore, a chemical equation is balanced when the number of each element in the equation is the same on both the left and right sides of the equation. Stoichiometry is the field of chemistry that is concerned with the relative quantities of reactants and products in chemical reactions. For any balanced chemical reaction, whole numbers are used to show the quantities of both the reactants and products.
For example, when oxygen and hydrogen react to produce water, one mole of oxygen reacts with two moles of hydrogen to produce two moles of water. Percent yield is the number calculated indicating the difference in percentage between the theoretical yield and actual yield of an experiment. When experimenting with different solutions or in the manufacturing of chemical solutions you have by-products and the actual intended products. The byproducts are what is left over and unusable after the production or experiment. In manufacturing using a chemical process, less by-product keeps the cost of production down as there is less waste. Percent yield will indicate how much product is left-over and help you formulate a plan to create less waste during the chemical reactions.
For more accurate measurements, glassware that has been certified by standards agencies may be purchased. Alternatively, one can calibrate glassware to an accuracy generally as good as the standards specifications, correcting for weight in vacuum, glassware expansion or contraction, and water expansion or contraction. Table 3.2-2 lists the calculated volumes for one gram of water in air at atmospheric pressure at sea level for different temperatures, corrected for buoyancy with stainless steel weights of density 7.8 kg m−3. The glass volumes are also calculated for the standard temperature of 20 °C, with small adjustments for borosilicate glass expansion or contraction with temperature changes. Is the volume occupied by one mole of a chemical element or a chemical compound. It can be calculated by dividing the molar mass by mass density (ρ).
Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. One way to determine the limiting reagent is to compare the mole ratio of the amount of reactants used. This method is most useful when there are only two reactants. One reactant is chosen, and the balanced chemical equation is used to determine the amount of the other reactant necessary to react with A. If the amount of B actually present exceeds the amount required, then B is in excess, and A is the limiting reagent. If the amount of B present is less than is required, then B is the limiting reagent.
In a chemical reaction, the limiting reagent, or limiting reactant, is the substance that has been completely consumed when the chemical reaction is complete. From stoichiometry, the exact amount of reactant needed to react with another element can be calculated. However, if the reagents are not mixed or present in these correct stoichiometric proportions, the limiting reagent will be entirely consumed and the reaction will not go to stoichiometric completion. The osmotic pressure of a solution is the minimum amount of pressure needed to prevent water from flowing into it across a semipermeable membrane. Osmotic pressure also reflects how readily water can enter the solution via osmosis, as across a cell membrane.
For a dilute solution, osmotic pressure obeys a form of the ideal gas law and can be calculated provided you know the concentration of the solution and the temperature. Such concentration calculations are needed when starting with the solid form of a chemical and a solution needs to be prepared with the concentration unit expressed in unit mass per unit volume (such as mg/mL). These calculations are especially useful when working with compounds that do not have well-defined molecular weights . The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law).
The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law). Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law). Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter.
Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures. A chemical equation is a visual representation of a chemical reaction. In a typical chemical equation, an arrow separates the reactants on the left and the products on the right. The coefficients next to the reactants and products are the stoichiometric values.
They represent the number of moles of each compound that needs to react so that the reaction can go to completion. The relationship between the products and reactants in a balanced chemical equation is very important in understanding the nature of the reaction. This relationship tells us what materials and how much of them are needed for a reaction to proceed. Reaction stoichiometry describes the quantitative relationship among substances as they participate in various chemical reactions.
Scientists sometimes use molality to measure concentration because liquid volumes change slightly based on the temperature and pressure. Mass, however, stays the same and can be measured accurately using a balance. Commercial concentrated products are usually expressed in mass percent; such as commercial concentrated sulfuric acid, which is 93-98% H2SO4 by mass in water .
The biggest issue when solving the problem is knowing the van't Hoff factor and using the correct units for terms in the equation. If a solution dissolves in water (e.g., sodium chloride), it's necessary to either have the van't Hoff factor given or else look it up. Work in units of atmospheres for pressure, Kelvin for temperature, moles for mass, and liters for volume. Watch significant figures if unit conversions are required.
Rearrangement reactions involve reorganization of the atoms of a molecule. To illustrate this, consider the acid catalyzed rearrangement of 3,3-dimethyl-1-butene to 2,3-dimethyl-2-butene . In this case the atoms of the reactant 12 are all shown in green since they are all incorporated into the desired product 13. As in the previous examples one can set up an atom economy table and calculate the % atom economy. As was predicted above for rearrangements, the % atom economy of this reaction is 100%.
Because addition reactions in general lead to the incorporation of all the atoms of the reactants into the final desired products, addition reactions result in high atom economy. From an atom economy point of view, addition reactions are thus environmentally preferable to elimination and substitution reactions. As a case in point consider the following addition of hydrogen bromide to methyl propene. In this example all the atoms of the reactants are shown in green since all of these atoms are utilized in the final desired product . The table of atom economy and the calculation of 100% atom economy further emphasize the excellent atom economy of this reaction.
How To Find Minimum Volume Chemistry Principle #5 prompts the consideration of auxiliary substances (solvents, separation agents, drying agents etc.) that are used in reactions and syntheses. It is thus clear that the waste generated from these auxiliary substances is significant and exceeds the amount of waste that is generated directly from the reaction. Many organic reactions utilize large amounts of organic solvents which are frequently toxic. These solvents often find their way into the water, soil and air resulting in significant pollution of the environment.
Efforts are underway to replace organic solvents with water, carbon dioxide and room temperature ionic liquids. In fact Joseph DeSimone of the University of North Carolina has been awarded a Presidential Green Chemistry Challenge award for his work in developingsurfactants for liquid and supercritical carbondioxide. As a result of Dr. DeSimone's efforts, a process using liquid carbon dioxide has been developed for the dry cleaning of clothes. This process recycles the carbon dioxide, that is obtained as waste from other chemical procedures and allows for the replacement of perchloroethylene, the health effects of which have come into question. Although the efficiency of a reaction can be measured in many ways, by far the most common way is to calculate the yield .
In general organic chemists consider yields of 90% or better as excellent while 20% or less are poor. Before carrying out any kind of lab work you need to to work out what is the theoretical yield, so you know how much of your product, be it a molecule or lattice , you can expect from a given amount of starting material. This allows you to work out how efficiently you carried out your reaction, which is done by calculating the percent yield.
The theoretical yield equation can also be used to ensure that you react equal moles of your reactants, so no molecule is wasted. To have value, measurement results must be metrologically traceable to an appropriate reference, which in the cases treated in this chapter are SI units of mass, volume, and amount of substance. A statement of measurement uncertainty always accompanies a traceable result. Methods must be validated and verified for use by a particular operator at a particular time. Accreditation to an appropriate standard, such as ISO , is overseen by organisations usually with governmental or quasi-governmental status.
Gaining accreditation for a particular method shows that a laboratory is using validated methods by competent personnel, but of course can never guarantee a reliable result . In this section we will review the components of measurement uncertainty of mass and volume measurements and then apply this to the preparation of a standard solution and a typical titration. It is noted that the metrological traceability chain will involve multiple branches , often through amount fraction or mass fraction. For more information, see the chapter on quality assurance in the forthcoming 4th edition of the Orange Book , or in . Chemical reactions, as macroscopic unit operations, consist of simply a very large number of elementary reactions, where a single molecule reacts with another molecule. As the reacting molecules consist of a definite set of atoms in an integer ratio, the ratio between reactants in a complete reaction is also in integer ratio.
A reaction may consume more than one molecule, and the stoichiometric number counts this number, defined as positive for products and negative for reactants . The unsigned coefficients are generally referred to as the stoichiometric coefficients. In general, chemical reactions combine in definite ratios of chemicals. Since chemical reactions can neither create nor destroy matter, nor transmute one element into another, the amount of each element must be the same throughout the overall reaction. For example, the number of atoms of a given element X on the reactant side must equal the number of atoms of that element on the product side, whether or not all of those atoms are actually involved in a reaction. To accurately calculate the yield, the equation needs to be balanced.
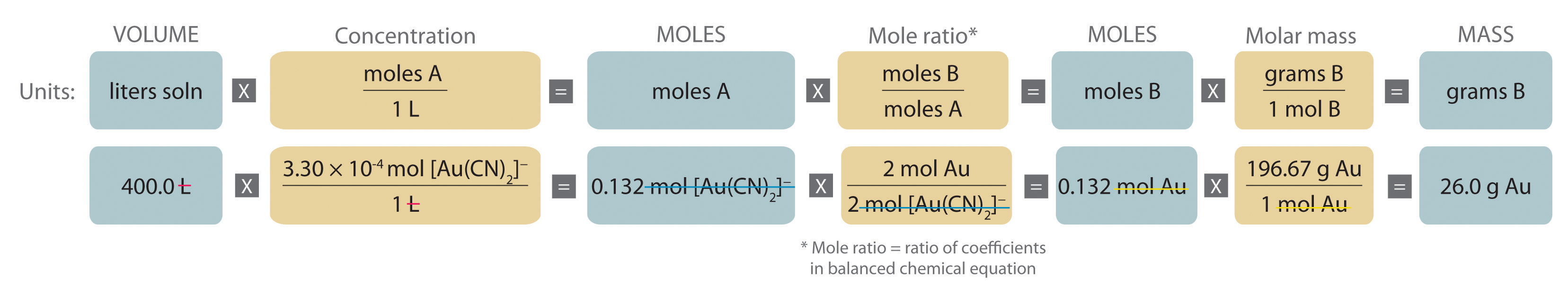
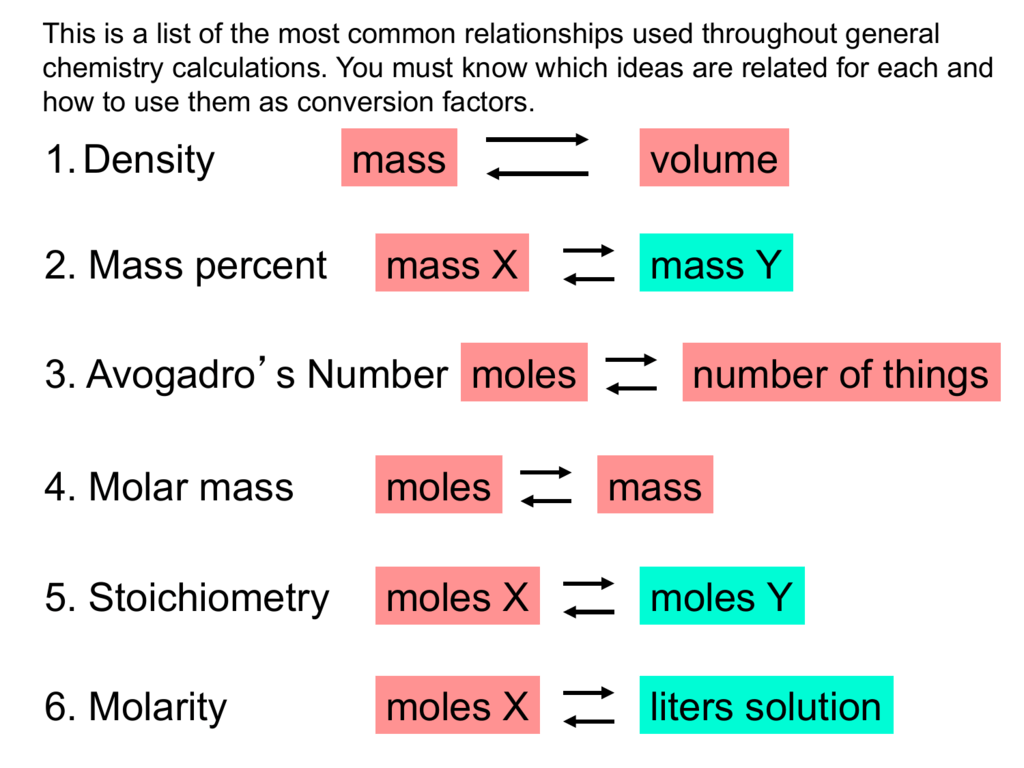
Then the theoretical yield of the product can be determined and, finally, compared to the actual yield. The balanced equation of a reaction contains the stoichiometric ratios of the reactants and products; these ratios can be used for mole -to-mole conversions. There is no direct way to convert from the mass of one substance to the mass of another.
4) If 0.502g of methane gas react with 0.27g of oxygen to produce carbon dioxide and water, what is the limiting reagent and how many moles of water are produced? Stoichiometry is a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. To make a solution from solid solutes, first calculate how many moles of solute are in the desired solutions . Calculate the amount of solid you need in grams using the moles needed and the molar mass of the solute and weight out the needed amount.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.